
Scientists Develop a Unique Model of the Human Small Intestine. It Could Replace Animal Testing
27. January 2026A scientific team from the Faculty of Technology (FT) and the Centre of Polymer Systems (CPS) at Tomas Bata University in Zlín, led by Professor Petr Humpolíček, is working in collaboration with the Institute of Biophysics of the Czech Academy of Sciences on a new three-year project funded by the Czech Science Foundation. The aim of the project, entitled EXIT & SIT: Ex Vivo Models of the Small Intestine, is to create an advanced laboratory model of intestinal tissue that faithfully replicates both the structure and function of the human small intestine.
A model that mimics reality
Modern biomedical research is increasingly replacing animal testing with so-called in vitro models – laboratory-engineered systems that simulate living tissues or organs. For simpler tissues, such as skin or the airway epithelium, such models are already widely available. However, for more complex organs like the small intestine, the development of these systems continues to face significant technological and biological challenges.
“The small intestine is an extremely complex tissue – it contains several cell types with diverse functions, and the cells themselves change depending on their position within the intestinal wall. On top of that, there are differences in the extracellular matrix, mechanical properties and the action of growth factors. Replicating such a system outside the body is demanding, but that is precisely what we aim to achieve with this project,” explains Professor Humpolíček.

Hydrogel scaffolds and the 3D villi structures
The foundation of the new model will be so-called hydrogel scaffolds – supporting materials structured into layers that mimic the individual parts of the intestinal wall. These layers will differ in composition, chemical and mechanical properties, and will be populated with specific cell types that form the intestinal lining. Using specially designed 3D-printed moulds, it will also be possible to create villi and crypts – characteristic structures of the small intestine – with micrometre precision.
Thanks to a unique layering technology and chemical cross-linking, the material will allow the creation of controlled gradients of growth factors, mechanical properties and extracellular matrix composition – key signals that regulate cell behaviour.
“Our goal is for the cells in the model to ‘think’ they are at home – in the correct layer, with the right support and the right signals. Only then can we obtain results that are truly reliable,” says Petr Humpolíček, the project’s principal investigator from the Faculty of Technology at TBU.
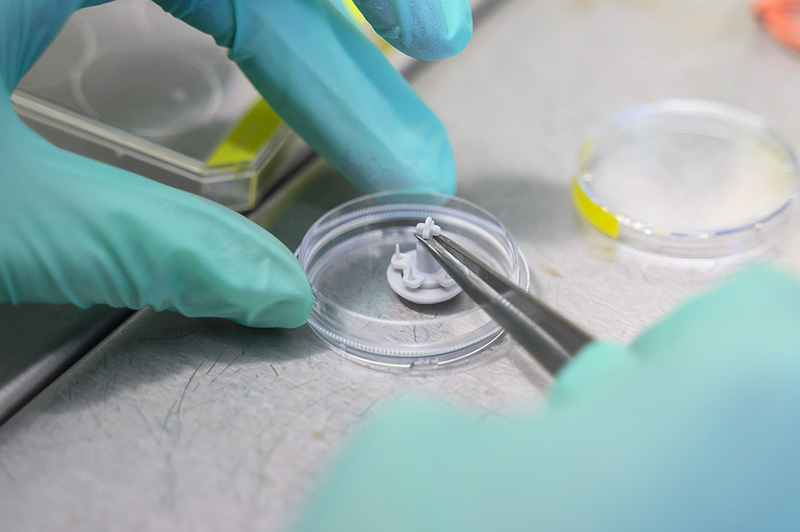
Not only cells, but also the microbiome
The project also involves a team from the Institute of Biophysics of the Czech Academy of Sciences, which will contribute its expertise in intestinal cells and the study of inflammatory diseases such as Crohn’s disease.
One particularly interesting aspect of the project is the effort to incorporate the intestinal microbiome – the naturally occurring microorganisms that play a crucial role in the health of the digestive tract. “We want to create a microbial biofilm on the surface of the model. Combined with microfluidics that will simulate the flow of intestinal contents, this will result in a complex system that is very close to reality,” adds Professor Humpolíček.
Applications in pharmaceuticals and medicine
The model under development could have a wide range of applications: in the pharmaceutical industry for testing new drugs, in research into inflammatory bowel diseases, as well as in nutrition and toxicology. As an ex vivo model that does not require the use of laboratory animals, it also has a significant ethical impact.
The project has been supported by the Czech Science Foundation with funding of CZK 11 million and will run until the end of 2027.
